
Atomic structure Mychem
Mass number. It is total number of proton and neutron present in the nucleus of each atom of an element. Mass number = No. of proton + no of neutrons. = atomic number - no of neutron. For example: the mass number of fluorine is 19 and atomic number is 9. Thus the number of neutron in an atom of fluorine is 19-9 =10.
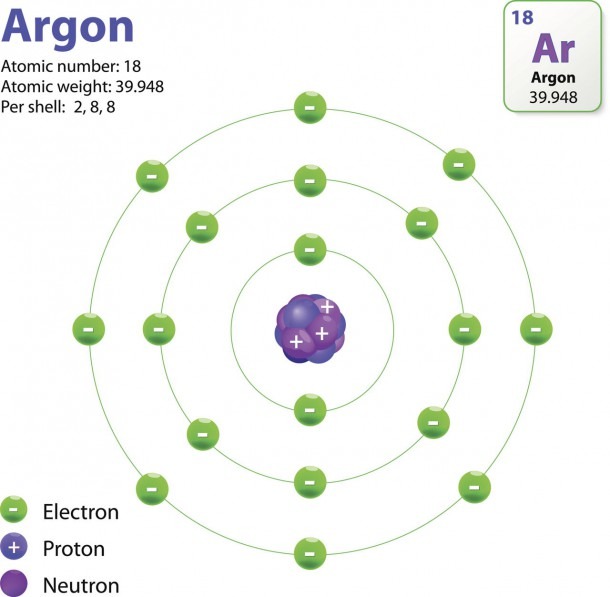
The Structure Of An Atom Explained With A Labeled Diagram Best
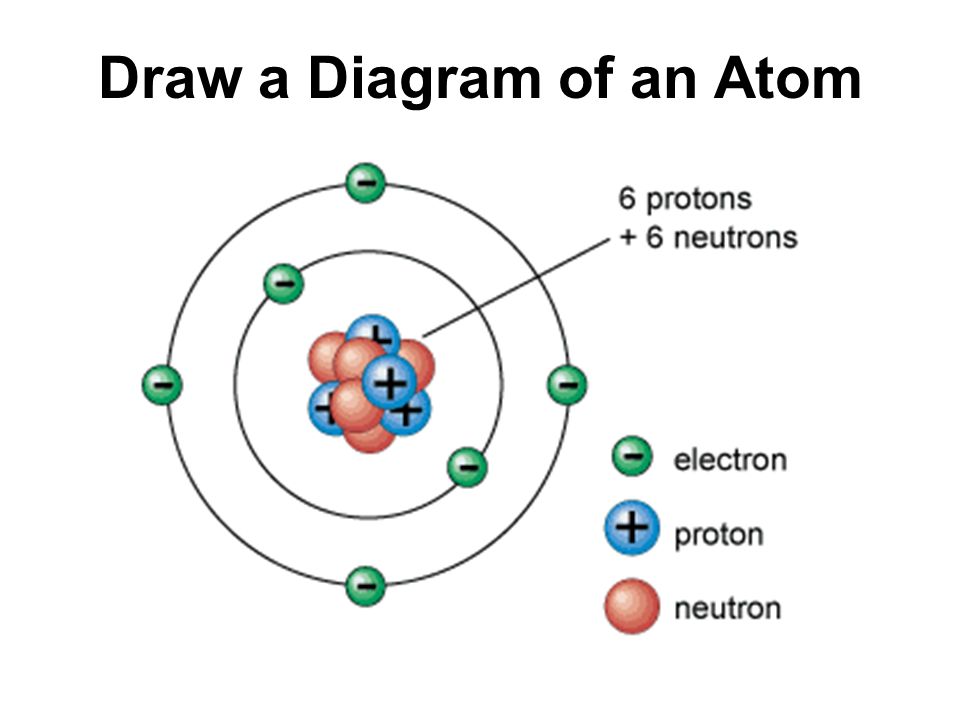
Draw your protons and neutrons. Erase the "C" in the center circle, and draw in your protons. Since protons are the same as the amount of electrons, you just draw 6 protons. To indicate they are protons, draw them as circles with plus signs contained inside. Neutrons are simply equal to the atomic mass minus the number of protons.
The Nucleus of the Atom and Radioactivity
As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: how likely it is to form bonds, and with which other elements. Electron shells and the Bohr model

Atom American Welding Society Education Online
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Skills Practice AMAZING 8TH GRADE SCIENTISTS
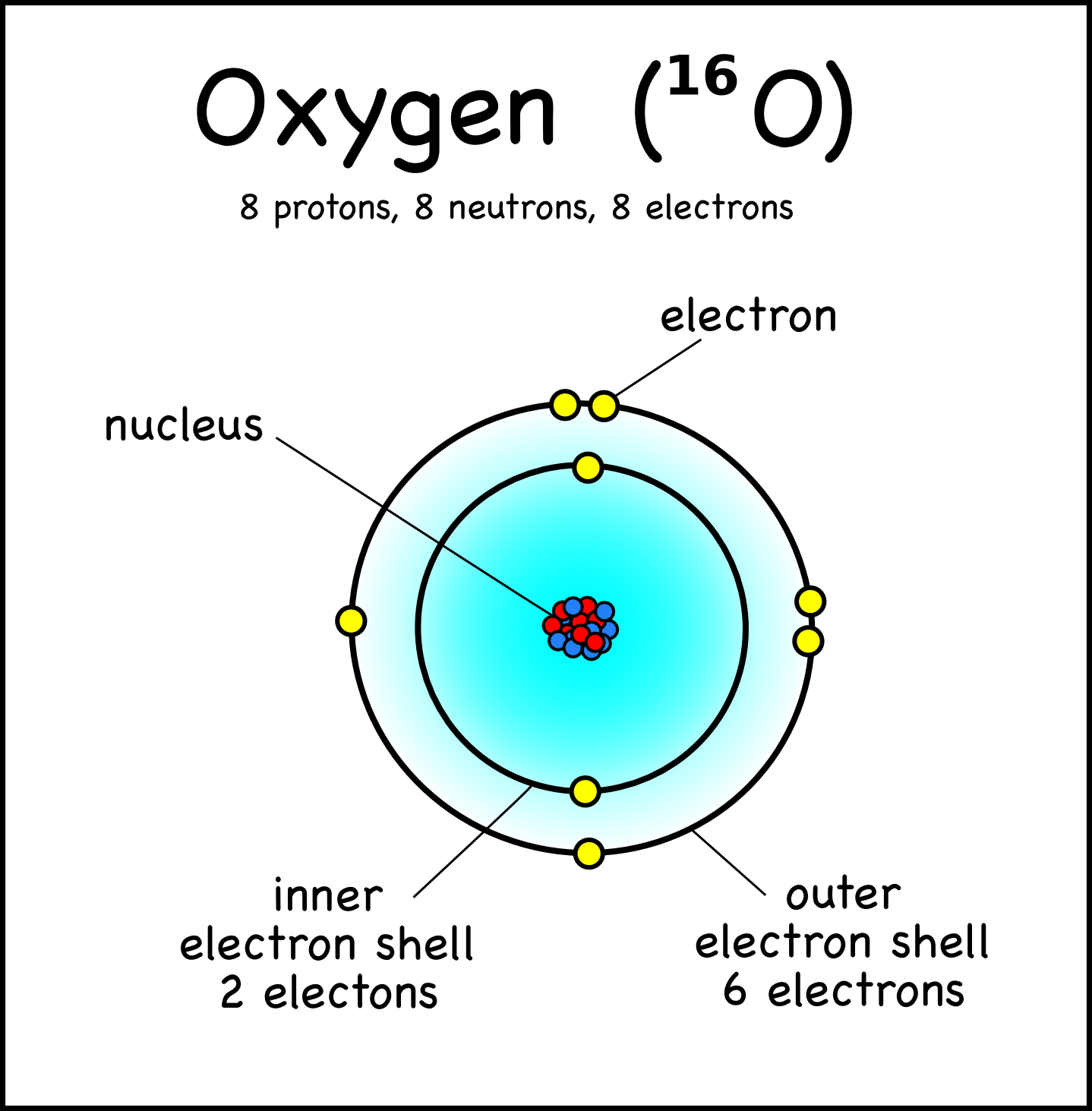
5.8: Orbitals. Page ID. Ed Vitz, John W. Moore, Justin Shorb, Xavier Prat-Resina, Tim Wendorff, & Adam Hahn. Chemical Education Digital Library (ChemEd DL) A characteristic of the diagram Figure 1 in Electron Waves in the Hydrogen Atom is that it has been assigned an identifying label, namely, 1 s.
Atoms and Atomic Structure HubPages
proton. Definition. Positively charged particle found in the nucleus of an atom. Location. Term. nucleus. Definition. The center of the atom which contains protons and neutrons. Location.

Learn the Parts of an Atom
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. When constructing a Lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms to gain, lose, or share.
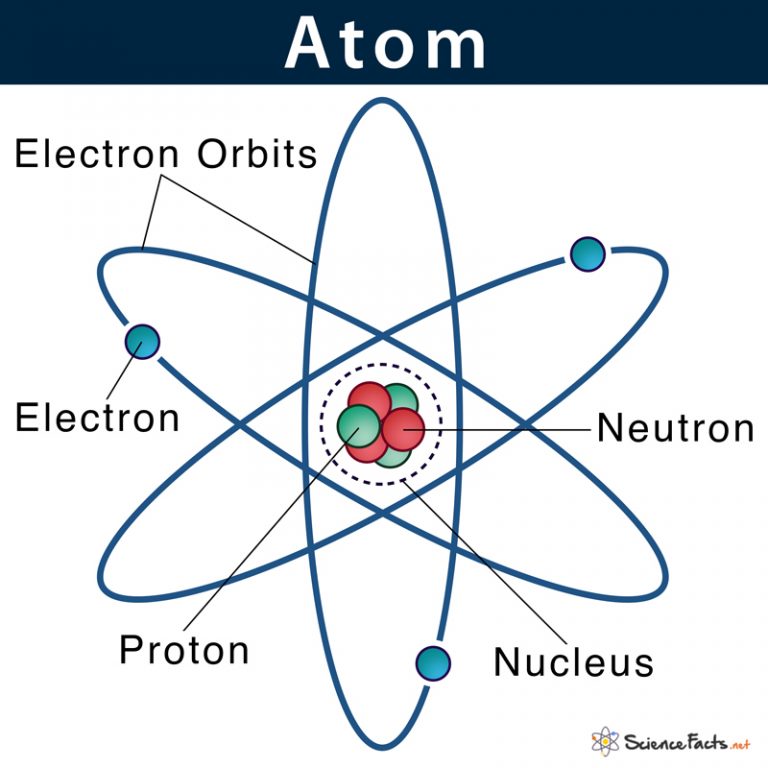
Atom Definition, Structure & Parts with Labeled Diagram
Negative particles in the electron cloud. Discovered by JJ Thomson. Particle with no charge in the nucleus of an atom. Discovered by Chadwick. Positive particles found in the nucleus of an atom. Discovered by Ernest Rutherford. Start studying Label the Atom. Learn vocabulary, terms, and more with flashcards, games, and other study tools.

Energy Mind Map
The Structure of an Atom Explained With a Labeled Diagram - Science Struck The Structure of an Atom Explained With a Labeled Diagram An atom is the basic unit of matter. The following article provides you with diagrams that will help you understand the structure of an atom better.

Label Parts of an Atom Teaching chemistry, Chemistry classroom
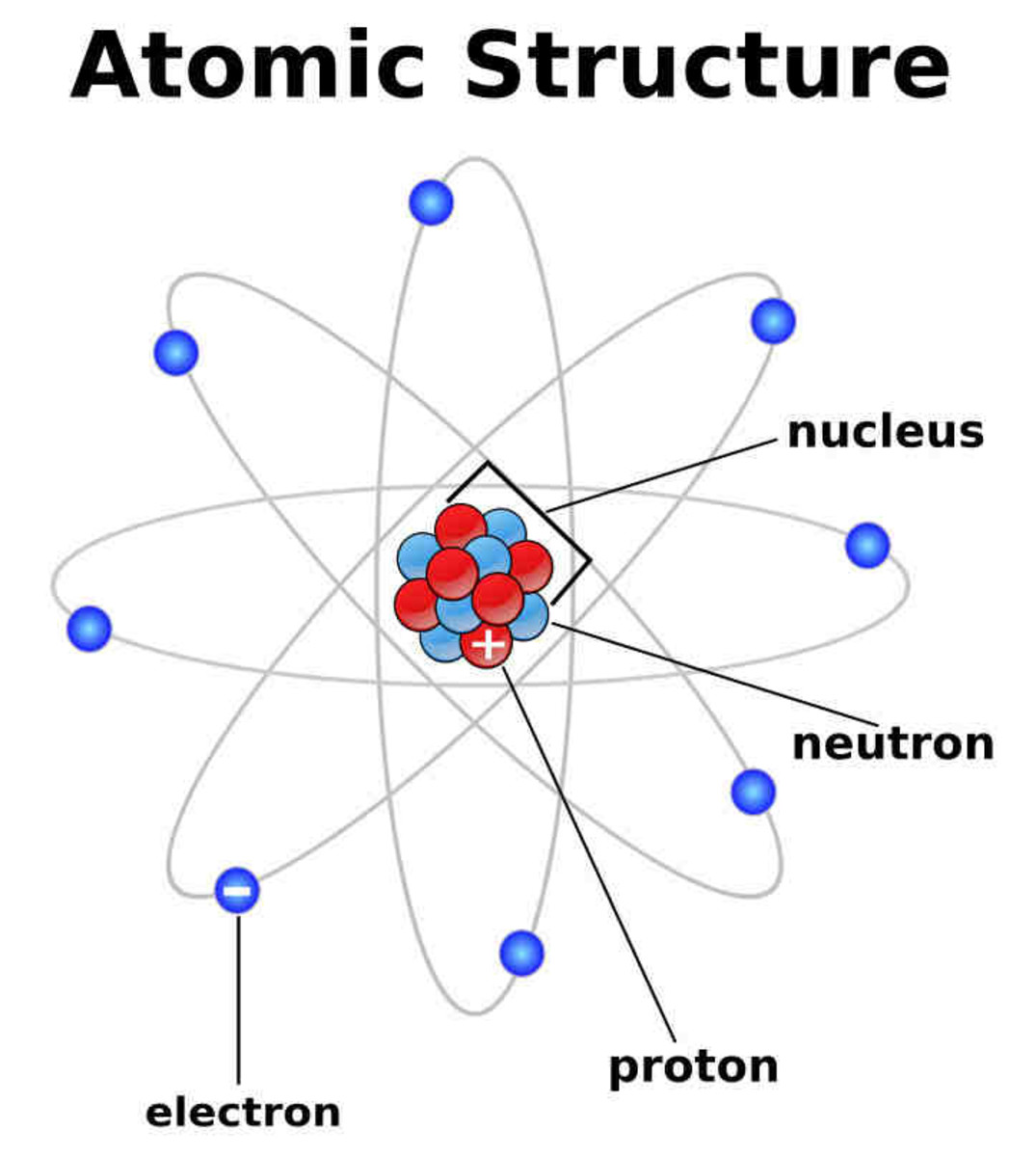
Structure of the atom Nucleus and shells An atom has a central nucleus . This is surrounded by electrons arranged in shells. The nucleus is tiny compared to the atom as a whole: the radius of.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
35 Label The Parts Of The Atom In The Diagram Below Labels For Your Ideas
Donate. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!

Biology 103 > Sizemore > Flashcards > BIO 103 Study Guide (201314
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of.

Atomic Structure Broad Learnings
Atom: Definition, Structure & Parts with Labeled Diagram Atom Atoms are tiny particles that form the basic building blocks of all matter in the universe, whether solid, liquid, or gas. All living organisms and nonliving objects found on Earth are made of trillions and trillions of atoms.
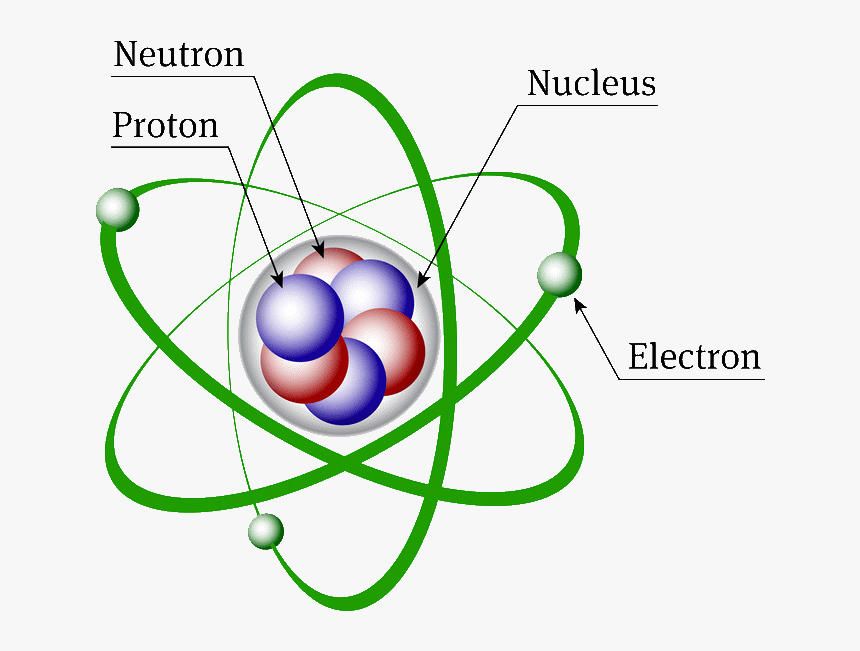
Structure Of An Atom Structure Of Atom Diagram, HD Png Download kindpng
Atomic diagrams were developed to explain the interaction of the elements of the Earth and space long before atoms could be observed. Nowadays, scientists can see particles that are smaller than.
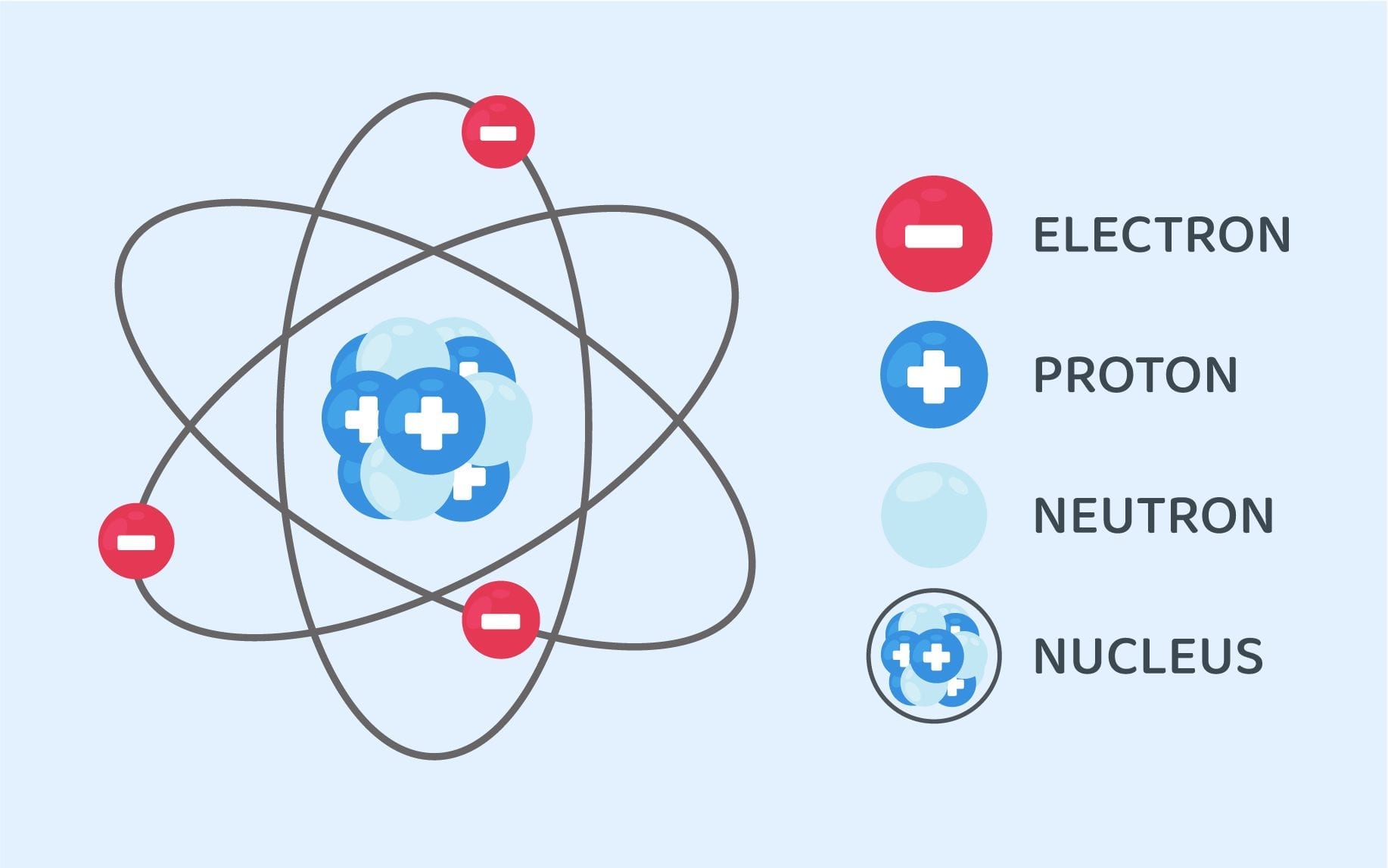
Structure Of An Atom Class 9 Science Notes Leverage Edu
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10 −10 metre. The radius of an atom measures 1-2 Å.
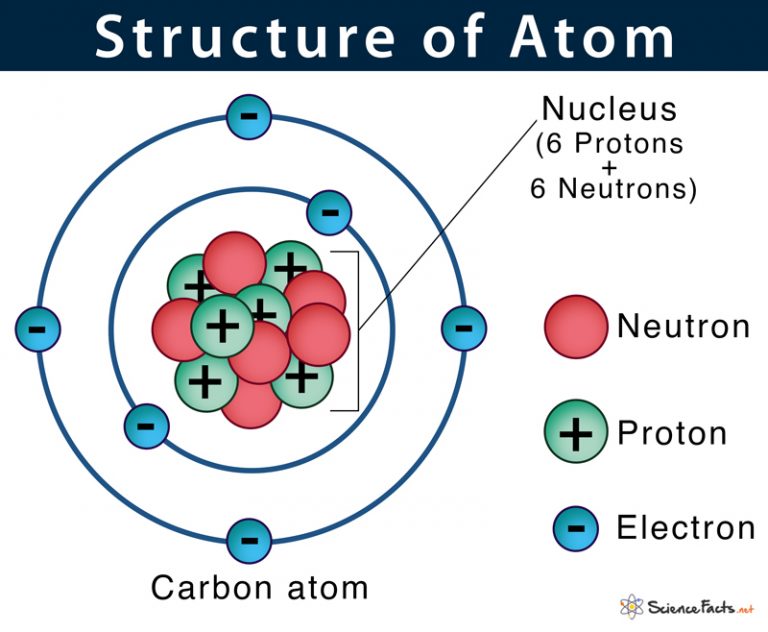
Atom Definition, Structure & Parts with Labeled Diagram
The Structure of an Atom: Parts, Diagram, Examples Anything that has mass and occupies space is called matter. The matter is made up of atoms. Atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral).